Refrigeration will not prevent growth and toxin formation by. In other words refrigeration is artificial human-made cooling.
Question 2 4 Pts You Are Designing A Refrigeration Chegg Com

Refrigeration Effect An Overview Sciencedirect Topics
In A Vapour Compression Refrigeration System Is The Heat Absorbed From The Environment It To Be Cold Bye Quora
A identify reasonably foreseeable hazards that could give rise to the risk.
Relation between absorbed heat and temperature in refrigeration. What is the relation between Q C Q G and Q E if they represent heat given to the refrigerant in the generator heat discharging to atmosphere from condenser to absorber and heat absorbed by the refrigerant in the evaporator. In order to manage risk under the WHS Regulations a duty holder must. Academiaedu is a platform for academics to share research papers.
1 In a transfer of energy as heat without work being done there are changes of entropy in both the surroundings which lose heat and the system which gains it. The comparison between R22 and NMR30 has been developed on the basis of Coefficient of Performance COP temperature at compressor exit power consumption per ton of refrigeration and volumetric refrigeration capacity variations when evaporation temperature and condensation temperature increase. Condensing temperature in a refrigerator is the temperature a of cooling medium b of freezing zone c of evaporator.
A short summary of this paper. A Q C Q G Q E b Q C. The kilocalorie kcal is a unit of energy measurement that represents the amount of heat needed to raise the temperature of 1 kilogram of water 1 C.
We would like to show you a description here but the site wont allow us. Expression for the Relation between C p and C. A heat pump is a machine or device that moves heat from one location at lower temperature to another location at a higher temperature using mechanical work or a high temperature heat source.
In a refrigeration system heat absorbed in comparison to heat rejected is a more b less c same d more for small capacity and less for high capacity e less for small capacity and more for high capacity. Since the mid-1960s there has been only a very slight increase in the warmest daily temperature Warmest Temperature graph above of the year amidst large inter annual variabilityHeat waves 6-day periods with maximum temperature above 90th percentile for 1961-1990 increased in frequency until the mid-1930s became considerably less. Mon Elvin B Jarabejo.
Heat is transferred from water drops to the surrounding air by the transfer of sensible and latent heat. The increased heat load causes the hot water temperature to increase considerably faster than the cold water temperature. A person conducting a business or undertaking must manage risks associated with using handling generating or storing of hazardous chemicals at a workplace.
This isothermal expansion is represented by curve 1-2 on p-v and T-s diagrams in the figure. In the refrigerator heat is absorbed at low temperature and rejected at higher temperature with the help of external mechanical work. Means that its population in relation to the mixed.
An assessment of the relative impact and risks connected with global warming. The important part of this definition is the removal of heat. Refrigeration may cause an increase in specific gravity and precipitation of amorphous phosphates and urates.
As the sample is in vacuum that is thermally isolated the. The air expands practically at constant temperature T1 from v1 to v2. Full PDF Package Download Full PDF Package.
B eliminate the risk so far as is reasonably. A closed urine transport system with integrated transfer tubes that contains preservative decreases the potential for bacterial contamination. The Harman method is known as a unique technique to measure thermal conductivity using the Peltier effect Harman 1958Suppose that a sample is hung by electrical leads in vacuum at a temperature TUpon a steady current I 0 the Peltier heat I 0 is generated at one current contact to the sample and is absorbed at the other.
Heat absorbed in the evaporator Work done by pump Heat supplied in the generator. The bars are colour-coded to show level of impactconcern for each factor as a function. Latent heat can be understood as energy in hidden form which is supplied or extracted to change the state of a substance without changing its temperature.
Assuming that a temperature difference of 111C exists between the working fluid and the river water on the one hand and the required room temperature on the other and assuming the heat pump to operate on the reversed Carnot cycle but with a COP of 50 of the ideal COP find the heat input to the engine per unit heat output from the heat pump. Heat is now moving from a. As nutrients move through the body they undergo ingestion digestion absorption transport cell metabolism and excretion.
The increase Δ S of entropy in the system may be considered to consist of two parts an increment Δ S that matches or compensates the change S of entropy in the surroundings and a further increment Δ S. Thermodynamics dictate that the heat removed from the water must be equal to the heat absorbed by the surrounding air. Latent heat also known as latent energy or heat of transformation is energy released or absorbed by a body or a thermodynamic system during a constant-temperature process usually a first-order phase transition.
The temperature difference between the two liquids is best utilized if they flow in opposite directions through the heat exchanger Figure 618. The process of removing heat from bodies colder than their surroundings is called refrigeration and the device doing so is called refrigerator. Glucose sensitivity is adversely affected by not testing at room temperature.
The term refrigeration means cooling a space substance or system to lower andor maintain its temperature below the ambient one while the removed heat is rejected at a higher temperature. 30 Full PDFs related to this paper. Refrigeration use the same principle but in reverse.
Heat Transfer 10thEdition by JP Holmanpdf. Five categories are assessed. The heat supplied the hot body is fully absorbed by the air and is utilized in doing external work.
The cold product then meets the cold heating medium at the inlet and a progressively warmer medium as it passes through the heat exchanger. It means that the temperature T2 at point 2 equals to the temperature T1. H at room temperature.
Alternatively heat 1 or 2 ml of enrichment. The Climate Science Special Report further notes.
Heat Pumps

Absorption Refrigeration System An Overview Sciencedirect Topics

Radiation Physics

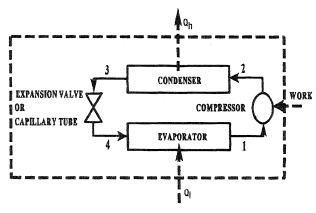
Refrigeration
Refrigeration Cycles Mech Engineering Thermodynamics Ucl Wiki
Refrigeration And Phase Changes

A Simple Guide To The Refrigeration Cycle And How Air Conditioners Work 2020 09 15 Achr News

Refrigeration Cycle Figure A 1 1 Heat Input And Output Of Vapor Download Scientific Diagram